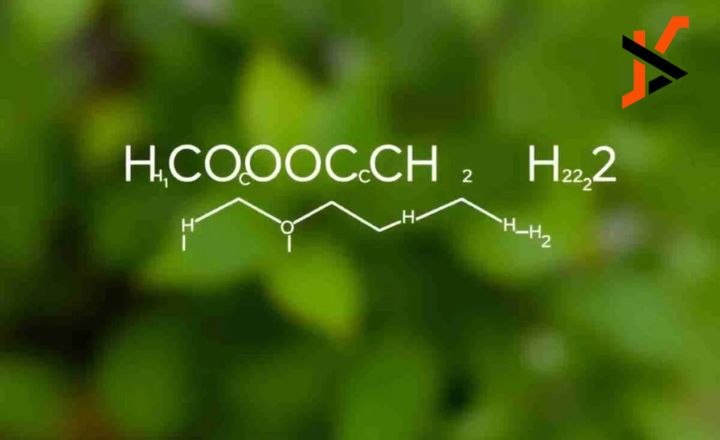
The chemical formula “HCOOCH CH2 H2O” encapsulates a fascinating interplay between molecular structure and chemical properties. This article will explore the significance of this formula, its implications in various scientific contexts, and its potential applications in industry and research. By breaking down the components, we can gain a deeper understanding of their relevance in chemistry and beyond.
What is “HCOOCH CH2 H2O”?
At first glance, “HCOOCH CH2 H2O” may seem like a complex chemical formula, but it represents a specific molecular structure that is critical in several chemical processes. To comprehend its significance, we need to analyze its components.
Breakdown of the Formula
The formula can be dissected into three main parts:
- HCOO: This portion represents the formate ion, which is the conjugate base of formic acid. Formate plays a crucial role in various biochemical reactions and is often involved in the metabolism of microorganisms.
- CH: This part signifies a carbon atom bonded to a hydrogen atom, contributing to the overall structure of the molecule. Carbon is a fundamental element in organic chemistry, known for its ability to form stable bonds with other elements, including itself.
- CH2: This fragment indicates another carbon atom bonded to two hydrogen atoms. The presence of these groups suggests that the compound may possess features common to alcohols or ethers.
- H2O: Water, represented here, is an essential component in numerous chemical reactions and is often a product or reactant in organic synthesis. Its inclusion indicates that the molecule may have hydrophilic properties, influencing its solubility and reactivity.
Potential Interpretation
When combined, “HCOOCH CH2 H2O” suggests a molecular structure that could represent a specific type of ester or ether, potentially involving a formate group and hydrocarbon chain. Understanding the precise arrangement of these atoms is crucial for predicting the compound’s properties and behavior in various environments.
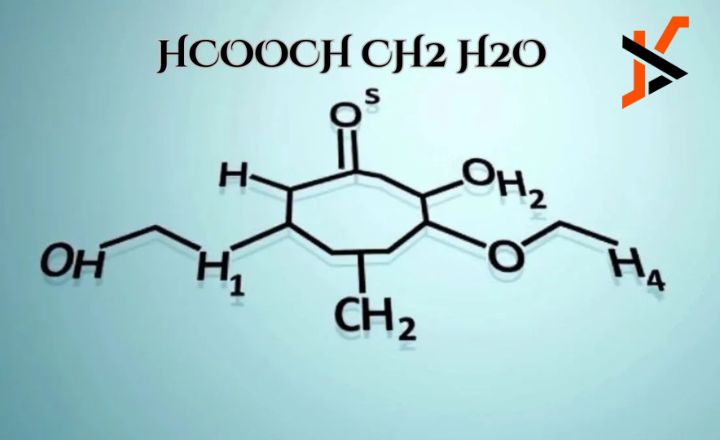
Applications of “HCOOCH CH2 H2O”
The implications extend across various fields, including organic chemistry, biochemistry, and environmental science. Here are some notable applications:
1. Biochemical Reactions
In biochemistry, compounds related to “HCOOCH CH2 H2O” play vital roles in metabolic pathways. For instance, formate is involved in the one-carbon metabolism, which is critical for synthesizing amino acids and nucleotides. Understanding how this compound interacts within biological systems can lead to advancements in metabolic engineering and synthetic biology.
2. Industrial Applications
In the industrial sector, derivatives may be utilized in the production of specialty chemicals, surfactants, and solvents. The hydrophilic properties contributed by the water molecule can enhance the compound’s solubility in various solvents, making it useful for formulating products in agriculture, cosmetics, and pharmaceuticals.
3. Environmental Impact
The study of compounds is also crucial in understanding environmental processes. Formate ions can be produced through the degradation of organic matter, playing a role in carbon cycling and the overall health of ecosystems. Investigating how these compounds behave in natural waters can provide insights into pollution control and bioremediation strategies.
A Comprehensive Analysis
The chemical formula encapsulates a fascinating interplay between molecular structure and chemical properties. This article will explore the significance of this formula, its implications in various scientific contexts, and its potential applications in industry and research. By breaking down the components,” we can gain a deeper understanding of its relevance in chemistry and beyond.
What is “HCOOCH CH2 H2O”?
At first glance, “HCOOCH CH2 H2O” may seem like a complex chemical formula, but it represents a specific molecular structure that is critical in several chemical processes. To comprehend its significance, we need to analyze its components.
Breakdown of the Formula
The formula can be dissected into three main parts:
- HCOO: This portion represents the formate ion, which is the conjugate base of formic acid. Formate plays a crucial role in various biochemical reactions and is often involved in the metabolism of microorganisms.
- CH: This part signifies a carbon atom bonded to a hydrogen atom, contributing to the overall structure of the molecule. Carbon is a fundamental element in organic chemistry, known for its ability to form stable bonds with other elements, including itself.
- CH2: This fragment indicates another carbon atom bonded to two hydrogen atoms. The presence of these groups suggests that the compound may possess features common to alcohols or ethers.
- H2O: Water, represented here, is an essential component in numerous chemical reactions and is often a product or reactant in organic synthesis. Its inclusion indicates that the molecule may have hydrophilic properties, influencing its solubility and reactivity.
Structural Interpretation
When combined, “HCOOCH CH2 H2O” suggests a molecular structure that could represent a specific type of ester or ether, potentially involving a formate group and hydrocarbon chain. Understanding the precise arrangement of these atoms is crucial for predicting the compound’s properties and behavior in various environments.

Area of Use
The implications extend across various fields, including organic chemistry, biochemistry, and environmental science. Here are some notable applications:
1. Reactions
In biochemistry, compounds related to it play vital roles in metabolic pathways. For instance, formate is involved in one-carbon metabolism, which is critical for synthesizing amino acids and nucleotides. Understanding how this compound interacts within biological systems can lead to advancements in metabolic engineering and synthetic biology.
2. Industrial usage
In the industrial sector, derivatives of “HCOOCH CH2 H2O” may be utilized in the production of specialty chemicals, surfactants, and solvents. The hydrophilic properties contributed by the water molecule can enhance the compound’s solubility in various solvents, making it useful for formulating products in agriculture, cosmetics, and pharmaceuticals.
4. Research and Progress
In research, “HCOOCH CH2 H2O” can serve as a model compound for studying reaction mechanisms and kinetics in organic chemistry. By examining how this molecule interacts with other reactants, chemists can gain insights into fundamental principles of reactivity and selectivity, paving the way for the development of new synthetic methods.
Conclusion
“HCOOCH CH2 H2O” represents a significant molecular structure with diverse implications across various scientific fields. Its components—formate, carbon, and water—highlight its relevance in biochemical reactions, industrial applications, environmental science, and research. Understanding this formula not only provides insights into its chemical properties but also opens avenues for further exploration in both academic and practical contexts.
As we delve deeper into the chemistry of compounds we recognize the importance of molecular structures in shaping our understanding of the natural world. This exploration underscores the interconnectedness of chemistry with biology, industry, and the environment, emphasizing the need for continued research and innovation in these areas. By appreciating the complexities of such compounds, we can better harness their potential for practical applications and scientific advancement.